As an emerging sterilization method, hydrogen peroxide sterilization has been widely applied in the pharmaceutical, medical, food, and other industries. Its significant advantages, such as high sterilization efficiency and easy degradability, make it an attractive option. As a result, it is gradually replacing traditional sterilization methods like ozone and formaldehyde. This raises the question: how was this effective and environmentally friendly sterilization method discovered?
In 1818, French chemist Louis Jacques Thénard first discovered hydrogen peroxide. Since then, using hydrogen peroxide aqueous solution (commonly known as hydrogen peroxide solution) for sterilization has gradually entered people’s daily production and lives.
In 1981, Steris Corporation of the United States found that hydrogen peroxide in a gaseous state has a spore-killing capacity at least 200 times stronger than that of liquid hydrogen peroxide or other sterilization methods.
Subsequently, in 1990, vaporized hydrogen peroxide (VHP) was officially approved by the U.S. EPA as a sterilizing agent for use in various industrial fields. In recent years, atomized hydrogen peroxide disinfection equipment has become a key sterilization device in high-level biosafety laboratories and has played an important role in multiple fields such as epidemic prevention and control, and hospital infection control. Previously, there had been ongoing debates at home and abroad regarding the sterilization efficacy of hydrogen peroxide. Internationally, the decontamination process of hydrogen peroxide vapor was regarded as “decontamination” rather than “sterilization”. It was not until January 8, 2024, when the U.S. FDA updated VHP from the established Class B sterilization process to the established Class A sterilization process—this signifies that the “sterilization efficacy” of VHP has been widely accepted.
Class A sterilization processes refer to those with a long application history, whose safety and efficacy are supported by information from multiple sources. They include dry heat sterilization, ethylene oxide (EO) sterilization, moist heat sterilization, and radiation sterilization.
Based on different principles, hydrogen peroxide sterilization methods are mainly divided into three categories.
Its main application scenarios include sterilization verification of sterility test isolators, sterile passboxes, and factory spaces. The sterilization process generally consists of three stages:
First, liquid hydrogen peroxide is converted into gaseous hydrogen peroxide through “flash evaporation” until the hydrogen peroxide saturation level in the test area no longer increases;
Maintain this state continuously to inactivate microorganisms; Finally, introduce fresh air to catalytically decompose hydrogen peroxide vapor into oxygen and water. The concentration of liquid hydrogen peroxide used ranges from 30% to 59%.

Tailin Sterility Test Isolator
This method works by using rotating airflow to spray liquid hydrogen peroxide, forming aerosols of 8–10 μm that disperse throughout the test area to inactivate microorganisms. It uses liquid hydrogen peroxide at a concentration of 5% to 8%, with a typical sterilization time of 2 to 3 hours. It is mainly applied in terminal disinfection and hospital infection control for high-level biosafety laboratories.
This method is primarily used with low-temperature hydrogen peroxide plasma sterilizers. The sterilization process proceeds as follows: First, liquid hydrogen peroxide is vaporized and injected into a vacuum sterilization chamber. Radio frequency (RF) or electrical energy is then applied to generate hydrogen peroxide plasma, which inactivates bacteria and viruses. Finally, the hydrogen peroxide plasma decomposes into water and oxygen.
This method uses liquid hydrogen peroxide at a concentration of 50% to 60% and features a relatively short sterilization time of 30 min to 120 min. It is widely used in hospitals and the medical device industry—primarily for the rapid sterilization of heat-labile precision medical devices such as artificial heart valves and cardiac pacemakers.
Hydrogen peroxide sterilization is a process influenced by multiple parameters, including hydrogen peroxide concentration, temperature, relative humidity, and saturation level. Due to the difficulty in fully controlling these parameters, there are no explicit standard documents specifying the D-value for biological indicators used in hydrogen peroxide sterilization efficacy validation.
Meanwhile, current technical methods make it challenging to scientifically and intuitively identify the worst-case conditions and locations for hydrogen peroxide sterilization. Therefore, the only feasible approach is to place a sufficient number of biological indicators in areas where hydrogen peroxide gas is difficult to diffuse, in order to conduct a sterilization challenge.
In general, sterilization efficacy validation involves aspects such as confirmation of sterilization conditions, selection of biological indicators, and the number of tests required.
The USP 2025 Edition states that given the differences in Vaporized Hydrogen Peroxide (VHP) sterilization cycle parameters, sterilization conditions must be confirmed based on historical experience. If the sterilization process parameters (typically time) fail to inactivate the biological indicators (BIs), these parameters should be adjusted until complete inactivation is achieved—this adjustment process is used to determine the minimum sterilization conditions.
PDA TR34 “Design and Validation of Isolator Systems for the Manufacturing and Testing of Healthcare Products” mentions the number of biological indicators to be placed during sterilization validation. In isolators, placing 5-10 BIs per square meter is sufficient. BIs should be dispersed at various locations within the aseptic isolator to confirm whether the diffusion level of the sterilization gas meets requirements.
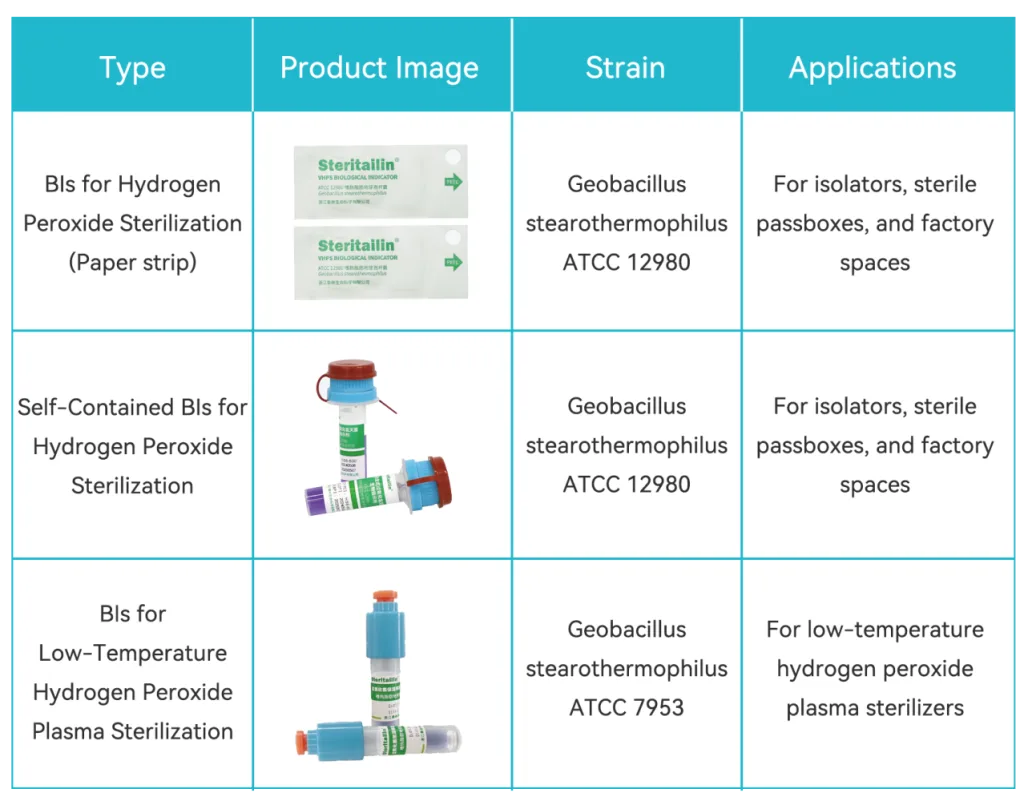
Based on different sterilization scenarios, Tailin has now launched three different types of hydrogen peroxide sterilization biological indicators to meet users’ diverse needs. Additionally, Tailin offers customized services for the spore count and D-value of various biological indicators, providing robust support for your sterilization validation.