In recent years, the results of FDA inspections on multiple pharmaceutical companies have repeatedly pushed the issue of microbial testing data integrity to the forefront, prompting the industry to re-examine: what kind of microbial testing data can be truly regarded as “complete”?
In November 2024, the FDA identified multiple serious cGMP violations (as outlined in 21 CFR 210/211) at the US-based E* Pharma. A warning letter issued in July 2025 classified several drugs as “adulterated” under Section 501(a)(2)(B) of the Federal Food, Drug, and Cosmetic Act, with microbial data integrity issues being particularly prominent. The letter cited:
- Incomplete/defective microbial data records (violating 21 CFR 211.194(a)): Lack of secondary verification for plate counts directly compromised data integrity and rendered Quality Assurance (QA) ineffective.
- Outsourced facility management failures (under Section 503B): No sterility testing per batch, and severe gaps in environmental monitoring – absence of real-time dual verification (only one analyst read plates), recording only positive results via photos in software, and immediate discovery of plate count errors during the FDA inspection.
- Additional systemic data management failures included lacking written procedures for process control (violating 21 CFR 211.100), no QC release (sign-off) records (violating 21 CFR 211.194(a)(8)), and incomplete visual inspection procedures (no defect library, insufficient risk assessment).
However, it is not an isolated case.
Behind these cases lie inherent challenges to data integrity in traditional microbial testing. Data Integrity is far more than just “not falsifying”; it requires data to meet the ALCOA++ principles—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, Available, and Traceable—throughout its lifecycle. However, in traditional testing, results cannot be automatically generated due to manual visual judgment and full manual operation relying on human experience; manual judgment and recording are prone to omissions and errors; the phenomenon of “backfilling” or “supplementing” records is difficult to eliminate; if key environmental data such as temperature, humidity, and differential pressure of equipment like incubators and sterilizers are not continuously recorded or are lost, the validity of test results will be directly affected, making “completeness” an empty promise.
So, where exactly is the boundary of “complete” in microbial testing data? The 2025 edition of the Chinese Pharmacopoeia General Chapter 9203 “Guidelines for Quality Management of Pharmaceutical Microbiology Laboratories” provides a clear answer: “Experimental records and data shall be true, accurate, complete, and traceable. Experimental records shall include all key experimental details to ensure the reproducibility of laboratory activities.” It also emphasizes that “each key experimental equipment used in the test shall have records; equipment logs or forms shall be reasonably designed to meet the traceability of test records. Equipment temperatures (water baths, incubators, sterilizers) must be recorded and traceable.”
Tailin’s Solutions for Data Integrity Management
01 Tailin Automatic Colony Counter Workstation SCW Series
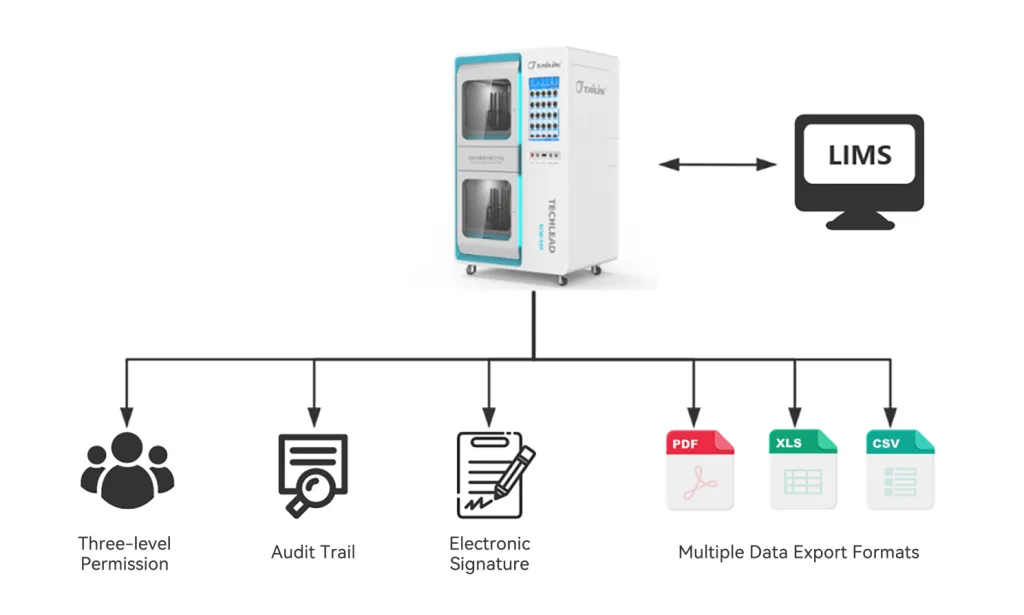
- Achieves batch rapid counting: colonies are detected and counted as soon as they grow, with full automated operation reducing the risk of errors caused by human intervention.
- Continuously counts and saves growth images, enabling dynamic playback of colony growth videos, output of colony count curves, and automatic generation of result reports—this full traceability feature meets the core requirements of data integrity.
- Adopts self-developed AI algorithms: through learning and training on massive data, it can real-time judge colony growth laws, confirm colony targets faster and more accurately, and eliminate interference from adherent colonies and impurities.
- Incubator environmental monitoring system: real-time monitors temperature and humidity, automatically alarms for abnormal fluctuations, ensuring stable culture conditions.
- Supports compliance functions such as three-level permissions, electronic signatures, and audit trails, meeting the requirements of 21 CFR Part 11. It facilitates data management and analysis for laboratory personnel and enables remote supervision.
02 Tailin Automatic Colony Counter ASC Series
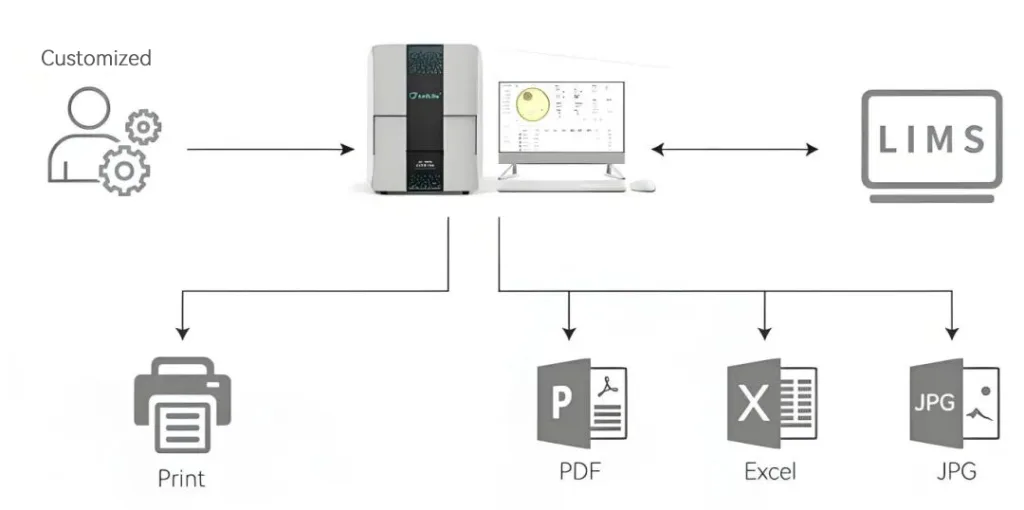
- Realizes full electronic process of sample photographing, result interpretation, and reporting.
- Operation records are automatically tracked, meeting data integrity requirements.
- Seamlessly interfaces with laboratory equipment (e.g., microscopes) to build an integrated microbial testing platform.
- Supports customer customization and LIMS system integration.
03 Tailin Automatic Rapid Sterility Test System AST Series
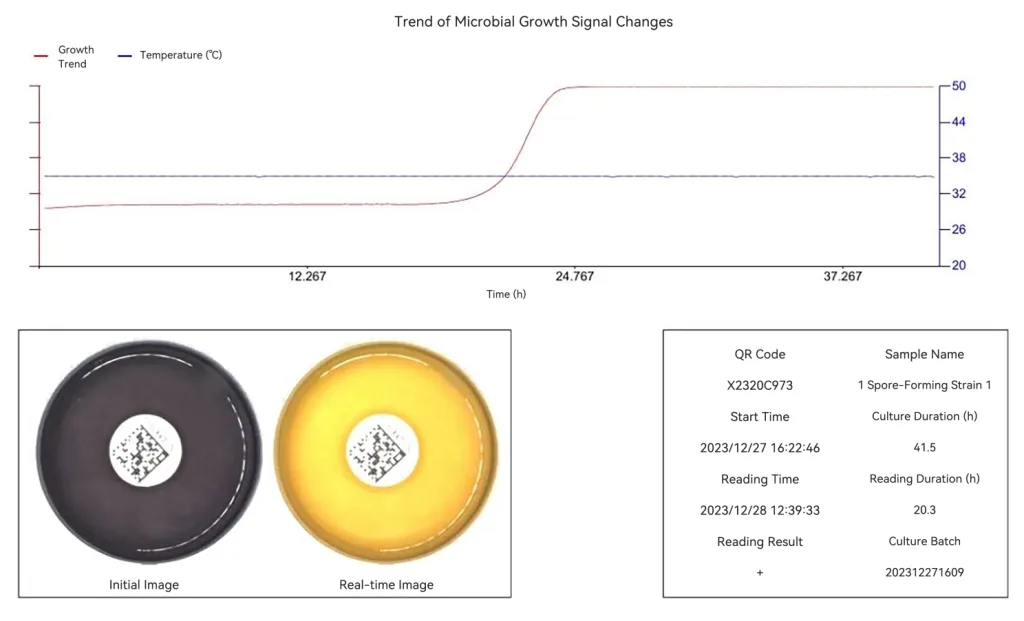
- Self-developed intelligent algorithms: adopts colorimetry + visual image analysis to avoid misjudgments caused by turbid samples.
- Automatically implements sample photographing, continuous monitoring, and result interpretation, with positive results available in as fast as 8 hours.
- Supports compliance functions such as three-level permissions, electronic signatures, and audit trails, meeting the requirements of 21 CFR Part 11. It facilitates data management and analysis for laboratory personnel and enables remote supervision.
In conclusion, data integrity is not just about compliance, but also a reflection of quality.
From vulnerabilities exposed in FDA warnings to explicit new Chinese Pharmacopoeia requirements, the industry’s understanding of “complete” microbial data is evolving. Data integrity is now a core focus for regulatory inspections and audits. Given the inherent complexities of microbial testing—subjectivity in manual interpretation, high error risk, and vulnerability of environmental data loss—companies must proactively build robust data management systems to avoid compliance crises.



