Home > Applications > QC Lab
QC Lab
Overview of
Microbiological Testing
Microbiological testing plays a vital role across life sciences and related industries, including pharmaceuticals, cosmetics, municipal water, and food & beverage, to ensure products are safe for use and consumption.
Tailin provides comprehensive microbiological testing solutions that meet international pharmacopoeia and GMP requirements.

Sterility Test System
Testing Environment and Compliance
Sterility testing should be conducted in an isolator system or a Class A unidirectional airflow clean area with a Class B background. To prevent microbial contamination, the entire process should strictly adhere to sterility testing. Cleanliness validation must be performed for both the unidirectional airflow area and the workbench.

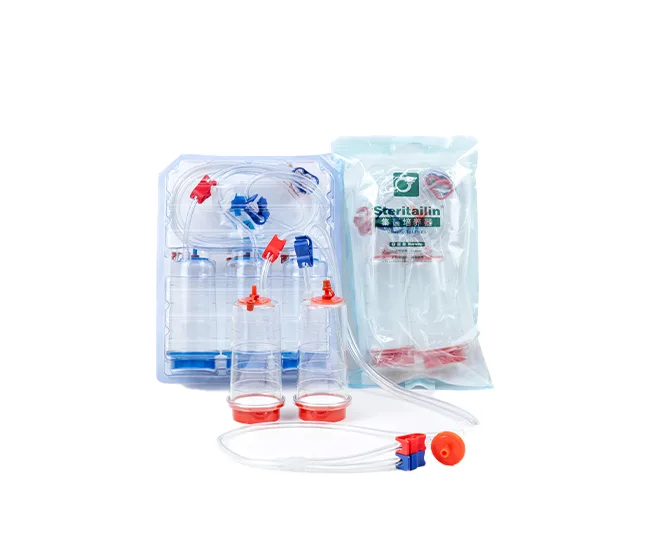
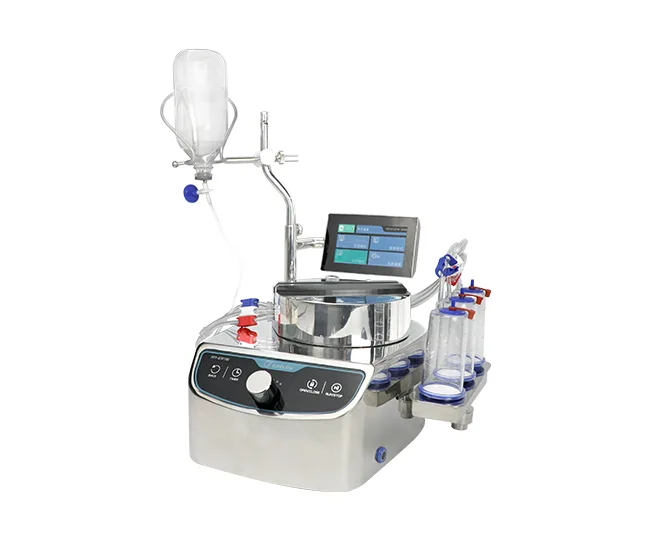
Tailin Sterility Testing Solutions
Tailin specializes in sterility testing research and provides a complete sterility test system that complies with the Chinese Pharmacopoeia, USP, EP, and JP.
Solutions include:
- Aseptic Isolator + Built-in Sterility Test Pumps & Canisters
- Sterility Test Pumps & Canisters
These products are widely used in pharmaceutical companies, QC laboratories, and microbiology facilities.
More
Microbial Limit Test System
Purpose and Principle
Microbial testing refers to the application of microbiological theories and methods to examine the types, quantities, and properties of microorganisms present in products such as food and pharmaceuticals, as well as their effects on human health. This process is used to determine whether a product meets quality standards.

Tailin Microbial Limit Test Solutions
Tailin microbial limit test system is based on membrane filtration technology, consisting of:
- Microbial limit testers
- Manifolds
- Funnels
- Membrane filters and accessories
More
Sterilization Monitoring and Validation—BIs & CIs
Tailin provides a wide range of biological indicators (BIs) and chemical indicators (CIs) to ensure effective microbial
control and regulatory compliance across pharmaceutical, medical device, and laboratory environments.