Home > Filtration Membranes > Flat Membrane > Reinforced PES Flat Membrane
The Reinforced PES membrane is hydrophilic by nature. It casts the polyethersulfone onto the support layer, giving it excellent mechanical strength and easy thermal bonding. It has excellent heat resistance, acid and alkaline resistance, very low protein binding, and low extractability.
Widely used in filtration applications in the fields of biopharmaceuticals, medical, water treatment, and chemical purification.
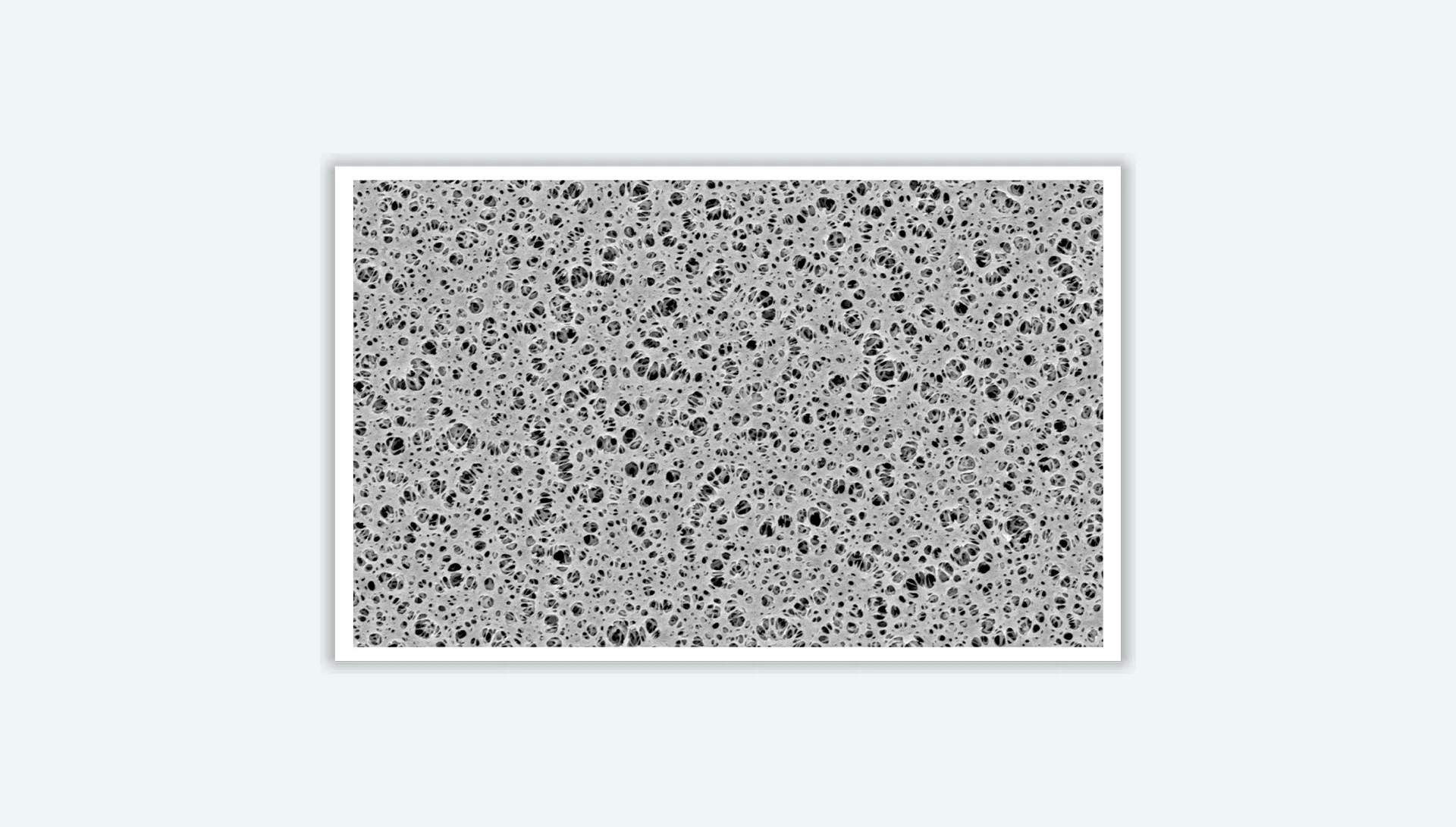
| TLPESB022254 | Type PES, Supported, Pore Size 0.22μum, Width 254mm, Rolling film |
| TLPESB022270 | Type PES, Supported, Pore Size 0.22μum, Width 270mm, Rolling film |
| TLPESB045254 | Type PES, Supported, Pore Size 0.45μm, Width 254mm, Rolling film |
| TLPESB045270 | Type PES, Supported, Pore Size 0.45μm, Width 270mm, Rolling film |
| Note: Other specifications can be customized (minimum order quantity requirements must be met) |
Suitable for process filtration in food & beverage, pharmaceutical, semiconductor, laboratory filtration, and water treatment.



