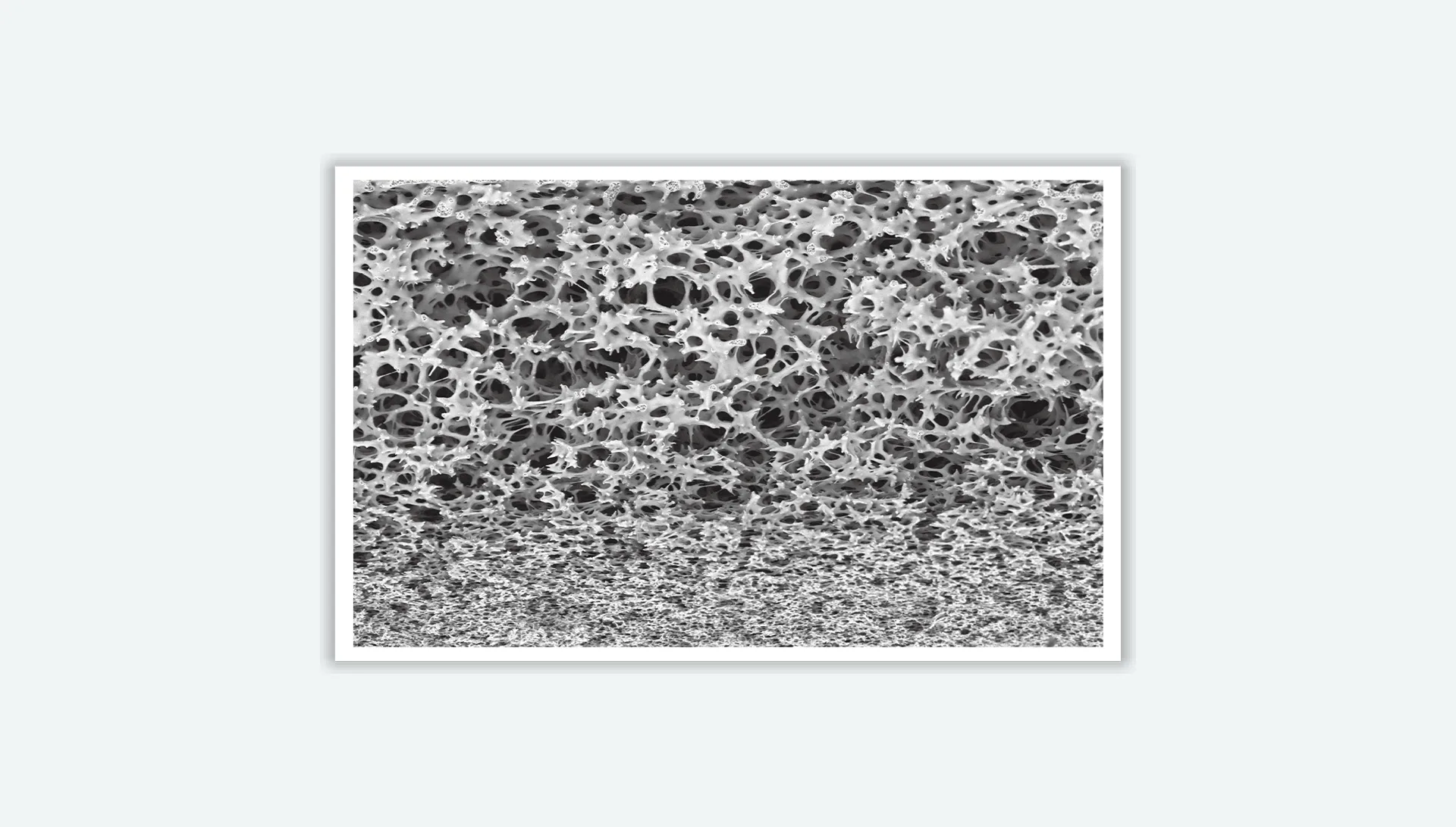
PES membrane is a natural hydrophilic, with good wettability and an asymmetric pore structure, so it has a super high water flux. PES membrane is known for its thermal stability, durability, and resistance to acidic and alkaline solutions. It shows very low protein adsorption and low extractable levels. Widely used in filtration applications in the fields of food and beverage, biopharmaceuticals, microelectronics, industry, water treatment, and laboratories.
| TLPES010270 | Type PES, Pore Size 0.1μm, Width 270mm, Rolling film |
| TLPES022270 | Type PES, Pore Size 0.22μm, Width 270mm, Rolling film |
| TLPES045270 | Type PES, Pore Size 0.45μm, Width 270mm, Rolling film |
| TLPES065270 | Type PES, Pore Size 0.65μm, Width 270mm, Rolling film |
| TLPES080270 | Type PES, Pore Size 0.80μm, Width 270mm, Rolling film |
| Note: Other specifications can be customized (minimum order quantity requirements must be met) |
Suitable for process filtration in food & beverage, pharmaceutical, semiconductor, laboratory filtration, and water treatment.



