
In the global field of cell therapy, technologies such as CAR-T and stem cell therapies are steadily maturing, positioning cell-based drugs as a new strategic high ground in the biopharmaceutical industry. However, despite continuous technological breakthroughs, the path to industrialization—from laboratory to market—remains fraught with challenges. How to overcome the “last mile” and realize the standardized, automated, and large-scale production of cell-based drugs has become a core issue urgently awaiting resolution.
The industrialization of cell therapies poses a unique set of challenges due to the following product characteristics:
Personalized Nature: Small production batches and highly stringent requirements for preparation and quality control lead to high costs;
Biological activity: Terminal sterilization is not feasible at any stage of production;
High Contamination Risk: Complex biological features, nutrient-rich cultivation processes, and strict environmental demands;
Short Shelf Life: Without prompt reinfusion or cryopreservation after preparation, product characteristics may degrade rapidly.
These factors impose strict requirements on manufacturing environments, operating procedures, and equipment reliability, all of which present major obstacles to industrial-scale production.
Last year, Mr. Sun Weiqun, Chief Expert of CGT Business at Tailin, was invited to deliver a keynote speech at the 2024 China Biopharmaceutical Biotechnology Annual Conference and Top 10 Advances Announcement. In his presentation, titled Large-Scale Preparation of Cell Therapies: From Lab to Commercialization, he offered a deep analysis of the current global landscape and challenges in the cell therapy sector. His insights helped experts and scholars at the conference recognize that the cell therapy industry is now undergoing a pivotal phase of accelerated transformation, particularly in bridging the gap between laboratory research, clinical application, and industrial-scale production.
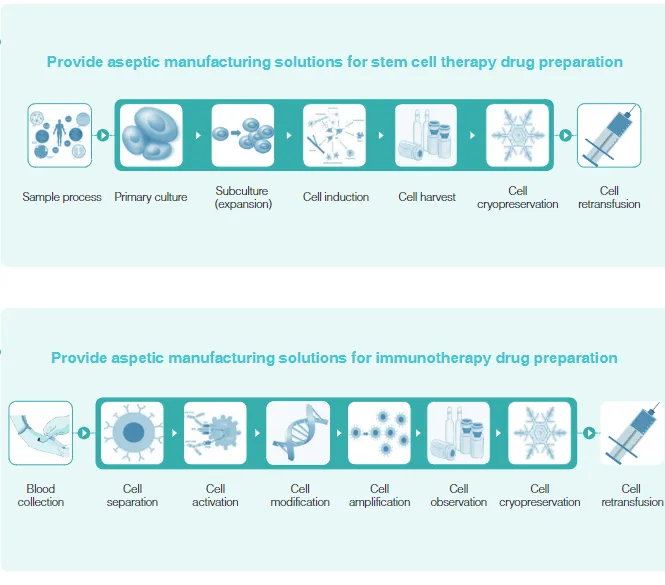
To overcome these bottlenecks, Tailin has introduced a comprehensive solution for CGT drug manufacturing:
Tailin has developed a fully enclosed Cell and Gene Therapy Isolator and a Honeycomb Cell Culture System, based on CGT drug manufacturing processes and GMP-compliant aseptic production requirements. These systems are designed to replace the traditional open “B+A” cleanroom environments commonly used in cell laboratories. They enable the execution of key processes such as cell separation, activation, modification, expansion, observation, collection, and filling, offering customers an innovative solution to support the commercial-scale production of CGT drugs and accelerate the industrialization of cell therapies.
The large-scale manufacturing of CGT drugs must be conducted in a sterile environment. This is particularly critical during key steps such as cell separation, expansion, and modification, where any contamination can lead to batch failure or pose serious risks to patient safety. In this regard, the Cell and Gene Therapy Isolator provides a reliable safeguard. Specifically designed for cell drug manufacturing, the isolator is a sealed operational system that employs advanced air filtration and airtight isolation features to ensure a highly sterile and controllable environment for cell handling.
High-Efficiency Sterile Environment:
Equipped with advanced filtration systems(HEPA or ULPA) to eliminate 99.999% of particles and microbes. Its sealed structure minimizes external interference during cell operations.
Flexible Process Adaptability:
Customizable for various production needs and compatible with a wide range of cultivation tools and equipment, enabling production of CAR-T cells, stem cells, and other immunotherapies.
Automation and High Efficiency:
Supports automated functions such as media changes and cell passaging, which significantly enhance efficiency, reduce manual error, and ensure consistency.
Compliance and Traceability:
Fully GMP-compliant with built-in monitoring and data recording systems for real-time tracking and full traceability throughout the production process.
Tailin is an integrated solution provider in life sciences. In the field of advanced therapy medicinal products (ATMPs), the company has established a comprehensive portfolio of automated, intelligent, and integrated solutions. Tailin collaborates with a wide range of partners, including cell and gene therapy companies, CROs/CDMOs, research institutions, and medical organizations. By delivering professional products and technical support, Tailin empowers the development and industrialization of biopharmaceuticals both in China and globally, accelerating the productization of cell and gene therapies.